Qualis LIMS Software Pricing, Features & Reviews
What is Agaram QuaLIS LIMS?
Agaram QuaLIS LIMS offers a range of solutions for any type of laboratory across multiple domains such as chemical, environmental, food and beverage, clinical research, pathology, public health, and pharmaceutical. It obeys the regulatory compliance requirements that laboratories need. This laboratory software complies with Good Laboratory Practice (GLP), Eudralex Annex 11, GCP, cGMP, Net Cord, 21 CFR Part 11, etc.
QuaLIS LIMS can be deployed on a web server and supports Databases and Open Source OS. It is basically used for Analytical purposes and QC/QA. This software, built on the Java platform, has some compelling features. Some of them are:
- Sampling and Storage: Users can manage sampling points in labs, receive samples and manage the custody of those samples.
- Specification Management: One can easily manage tests, samples, MDL, Formulae, etc. by using Agaram QuaLIS LIMS.
- Results: After the conduction of an experiment, results are published after going through two levels of review approval.
What are the features and technical highlights of Agaram QuaLIS LIMS?
Solutions
Agaram QuaLIS LIMS offers a wide range of solutions for:
- Document Management
- Stability Management
- Quotations and Invoicing
- Non-conformance (OOS / Deviations)
- Email Alerts
- Training Management
- Synthesis Project Management and Formulations
- Method Development and Validation
Technical Highlights
Basically, the structure of Agaram QuaLIS LIMS is classified into three parts:
- Lab - The lab consists of a PDA, RS232 Instrument and an RS232 to TCP/IP Converter.
- Data Center - It is exclusively composed of the QuaLIS Database Server.
- Local Area Network - The LAN comprises PC-Based Instruments, App Server and the QuaLIS Web Server.
Some of the salient technical points pertaining to the software are:
- The software is a web-based internet application, built using JAVA, HTML5 and JavaScript on a UI site.
- Agaram QuaLIS LIMS is specifically designed for multi-department and multi-site organizations.
- This web-based solution is accessible from browsers such as Chrome, Internet Explorer, etc.
- The software supports PostgreSQL and MS-SQL.
Pricing of Agaram QuaLIS LIMS
The pricing of Agaram QuaLIS LIMS is completely customizable and is available on the request of users. Drop-in a request to our website through the “request a callback” option and our customer care team will correspond with you shortly. We will support you with all possible help regarding cost, activation and license key, and renewal procedures.
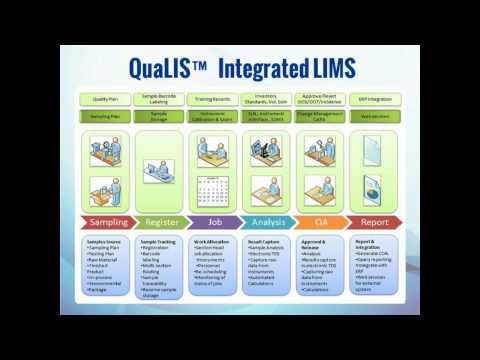
Agaram QuaLIS LIMS Data Workflow
The data workflow in Agaram QuaLIS LIMS follows a set of steps:
- Masters, Workflow: This is the planning process of an experiment. This step comprises rights and roles, tests, methods, sampling points, specifications and workflows.
- Approve / Reject Re-Test: This step is meant for checking, approving or ordering re-tests, reviewing, releasing reports and recording incidents.
- Sample Barcode Labelling: This is for sample storage and tracking. The process involves sample storage registration, sample routing, barcode registration and addition of the removed tests.
- Training Records Checking: It is used primarily for instrument calibration and maintenance. Through this step, one can receive samples and allot jobs for personnel and instruments, as well as for rescheduling or monitoring.
- Inventory Tracking: This procedure is used to calculate results with insightful analytics. One can also calculate inventory usage and generate a report by virtue of QuaLIS LIMS.






20,000+ Software Listed
Best Price Guaranteed
Free Expert Consultation
2M+ Happy Customers